BioAxone has created novel, innovative drugs with potential to transform the lives of patients.
Rho inhibitor (BA-210): A protein drug that is delivered locally to the site of spinal cord injury. Phase 3.
ROCK inhibitor (NRL-1049): Our orally available ROCK2 inhibitor is licensed to Neurelis. NRL-1049 (previously BA-1049) is drug candidate to treat cerebral cavernous malformation (CCM) and other diseases of blood brain barrier dysfunction.
PTEN inhibitor (BA-434): A self-delivering RNAi that targets axon regeneration and treatment of spinal cord injury.

Spinal Cord Injury
The first-line treatment for acute spinal cord injury is decompression surgery. Clinical research by Dr. Michael Fehlings at University of Toronto demonstrated that early surgery within the first 24 hour has the greatest benefit .
.
BA-210 delivered to the site of injury in a fibrin sealant
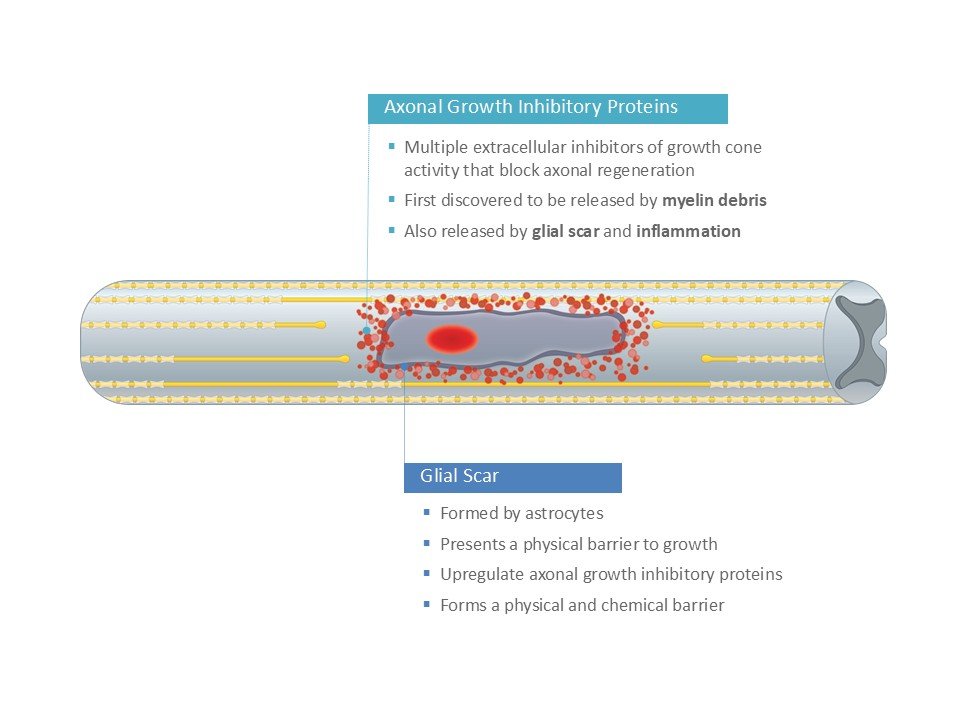
Dr. Lisa McKerracher (then at McGill University) led the first group to purify and identify a growth inhibitory protein in the central nervous system, the first of many. BioAxone took an innovative strategy to develop BA-210, a compound that targets Rho, a central convergence point in the signaling pathways activated by many different CNS inhibitory proteins.
BA-210 is used acutely to limit secondary damage and is delivered to the site of injury during surgical decompression.
History of BA-210
he technology, originally developed in Canada through a university spin-out, was transferred to the U.S. company, BioAxone BioSciences. Vertex Pharmaceuticals licensed BA-210 and initiated a Phase 2b/3 clinical trial. When Vertex later shifted its strategic focus to genetic diseases, the trial was halted following an interim analysis. While the study did not meet criteria to continue, signals of efficacy were observed in the 3 mg dose group.
All studies to date consistently demonstrate the importance of inactivation of Rho for neuroprotection and regeneration. Better clinical outcome measures are now available for refinement of outcome measures in future clinical studies.
Cerebral Cavernous Malformation
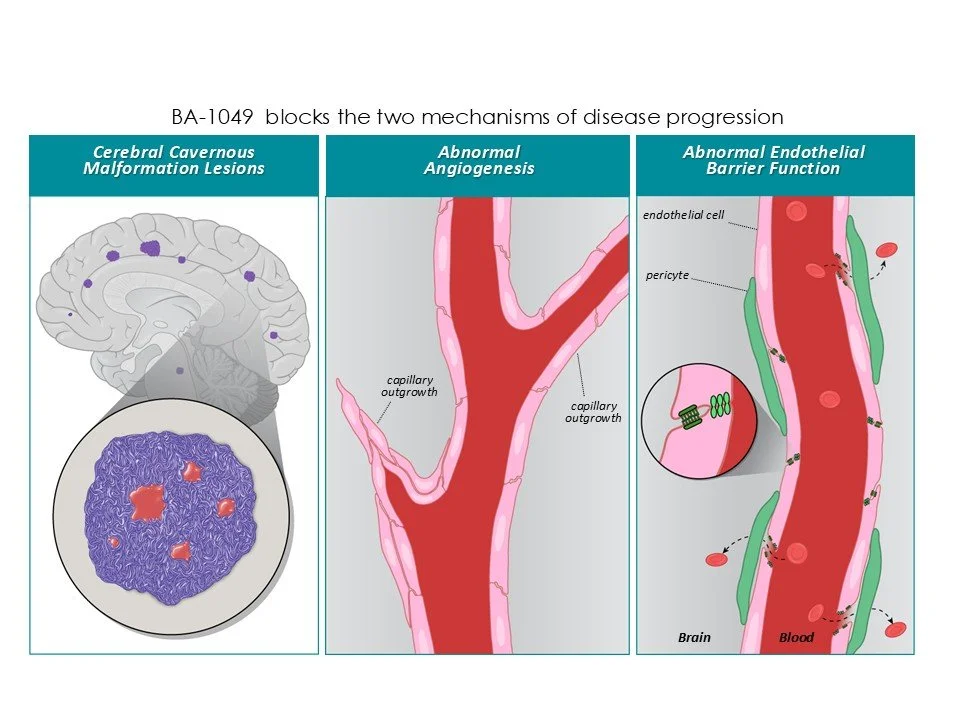
Cerebral cavernous malformation (CCM), also called angioma, is an inherited disease caused by a loss of function of one of the 3 CCM genes (CCM1, CCM2 and CCM3). Spontaneous lesions may also form. Approximately 200,000 Americans have had an incidence of bleeding in the brain from this disorder. Studies of the inherited mutations underlying angioma formation revealed that ROCK hyperactivation disrupts endothelial barrier integrity.
There are no drugs that prevent or reverse CCM lesion formation. The only treatment of symptoms is brain surgery, potentially causing additional neurotrauma.
Studies of CCM lesions from patients demonstrated that ROCK is hyperactivated in the endothelial cells. The loss-of-function mutations that cause CCM disrupt ROCK signaling, and this results in leaky cells that are prone to bleed in the brain.
Our small molecule drug candidates that target ROCK are ROCK2-selective, the form of the kinase most highly expressed in the CNS. BA-1049 reduces leakiness of endothelial cells and reduces lesion formation in animal models of the disease.
Success with BA-1049 for treatment of CCM will have relevance to other neurological diseases that include stoke and neurodegenerative disease where the blood brain barrier is compromised.
BA-1049 has been licensed to Neurelis and is now called NRL-1049. It is in Phase 1 clinical trials.
BA-434
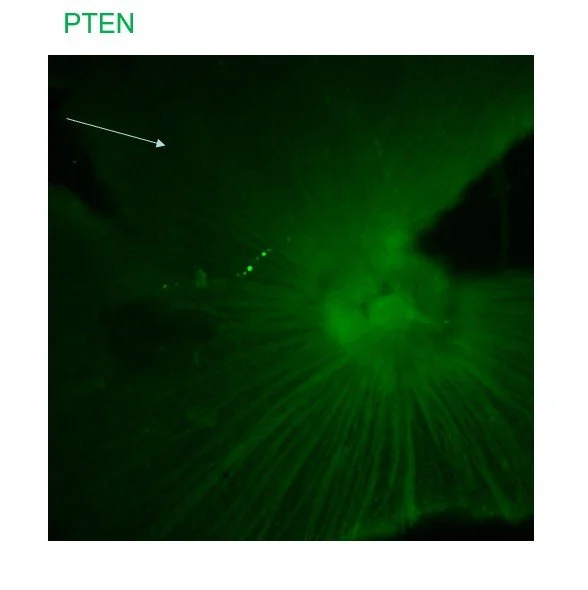
Developed for treatment of spinal cord injury, BA-434 targets PTEN, a well-validated target to promote axon regeneration. BioAxone created a self-delivering RNA interference compound that reduces expression of PTEN, a protein that blocks axon regeneration in adult neurons. This compound has shown efficacy in preclinical models of optic nerve injury.
PTEN is expressed in retinal ganglion cell axons
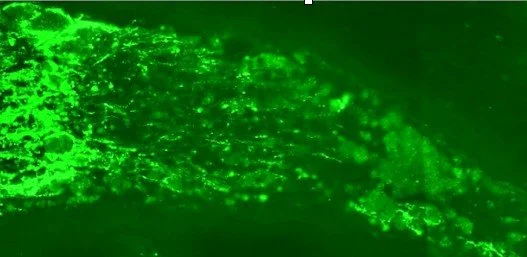
BA-434 silences PTEN in the retina
Regeneration in the optic nerve
PTEN silenced
No treatment